40 fda health claims on food labels
Health claims on food labels - PubMed Health claims on food labels Food and drug law requires that the ingredients in most foods be disclosed on their labels, but until recently there was no requirement that nutrition information be provided. The Nutrition Labeling and Education Act of 1990 (NLEA), passed on November 8, 1990, mandated the Food and Drug Administrati … Authorized Health Claims That Meet Significant Scientific Agreement Authorized Health Claims That Meet the Significant Scientific Agreement (SSA) Standard Authorized health claims in food labeling are claims that have been reviewed by FDA and are allowed on food...
Questions and Answers on Health Claims in Food Labeling | FDA All health claims, whether authorized or qualified, require pre-market review by the FDA. Under federal law, the FDA approves by regulation authorized health claims for use in food labeling only if...

Fda health claims on food labels
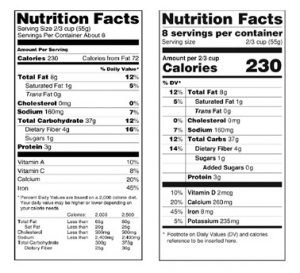
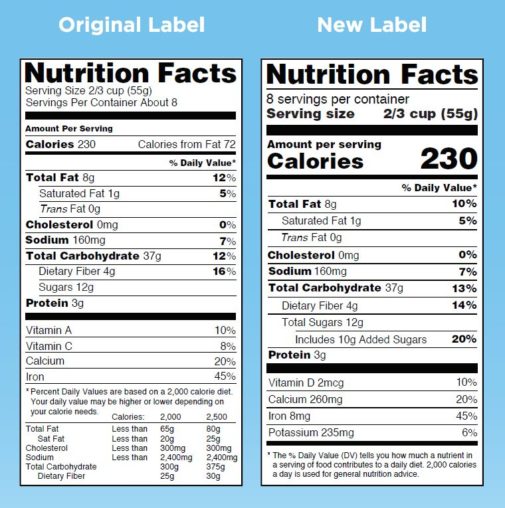
Legal Guide to Health Claims on Food | Law@Dayton The Nutrition Labeling and Education Act, which amended the FD&C Act in 1990, requires most foods to be labeled with serving sizes and specific nutrition information, and it sets standards for food labels that make certain health claims. The Fair Packaging and Labeling Act of 1966 spells out packaging requirements for food and other packaged ... Nutrient Content Claims | FDA - U.S. Food and Drug Administration Nutrient Content Claims. See Claims That Can Be Made for Conventional Foods and Dietary Supplements for definitions of claims. Final Rule: Food Labeling: Nutrient Content Claims; Alpha-Linolenic ... Factual Food Labels: Health Claims - University of Texas at Austin Generally, these labels are found on the front side of the food package in emphasized lettering. Health Claims. In 1990, the Nutrition Labeling and Education Act allowed claims that related a specific food component (e.g., oats) to lowered disease risk (e.g., reduced cholesterol) to be printed on the label of a food product. For example, if a ...
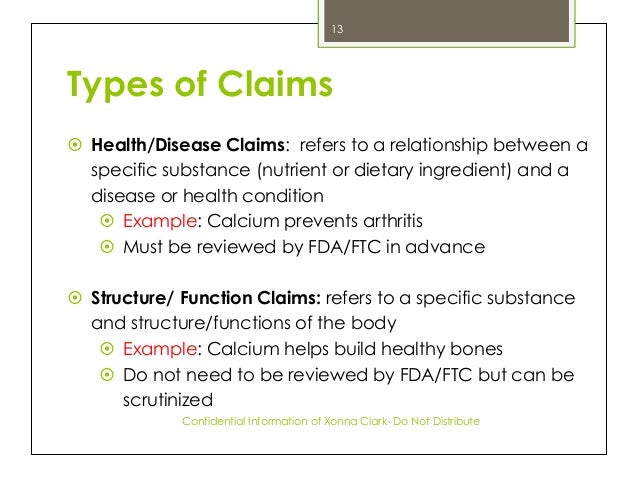
Fda health claims on food labels. Health Claims on Food Labels - LabelCalc Health claims, according to the FDA, are statements about the relationship between a food product or ingredient and a reduced risk of disease or a health condition. Basically, the FDA distinguishes two kinds of health claims: "authorized" and "qualified." Authorized Health Claims: Claims that have significant scientific agreement (SSA ... A Guide to FDA Regulation of Food Labeling Claims Among the FDA-regulated claims commonly declared on food labels are nutrient-content claims, health claims, qualified health claims and structure/function claims. Additionally, FDA has authority over claims related to gluten content, genetically modified organisms (GMOs) and "natural." Label Claims for Food & Dietary Supplements | FDA Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims,... Food Packaging Claims | American Heart Association It's important to understand what these claims mean so you can make informed decisions about the food you buy for yourself and your family. There are three categories of claims defined by statute and/or FDA regulations that can be used on food and dietary supplement labels: health claims, nutrient content claims, and structure/function claims.
Food Label Claims: What You Can and Can't Trust - WebMD The FDA is updating its definition for this claim. Until then, companies can make the "healthy" claim if the fats in their foods are mostly mono- and polyunsaturated fats. The healthy claim also... FDA perspectives on health claims for food labels - PubMed FDA perspectives on health claims for food labels Abstract The U.S. Food and Drug Administration's regulatory authority over health claims was clarified in 1990 legislation known as the Nutrition Labeling and Education Act (NLEA). CFR - Code of Federal Regulations Title 21 - Food and Drug Administration (1) health claim means any claim made on the label or in labeling of a food, including a dietary supplement, that expressly or by implication, including "third party" references, written statements... FDA Working to Replace Misleading Food Labels | Live Science Food labels on package fronts, which have proliferated and are seen as misleading, have come under FDA scrutiny. ... being considered and studied by the FDA in their effort to come up with new regulations governing front-of-package labels. (Image credit: FDA) ... as health problems associated with poor diets, such as heart disease and obesity ...
What You Need to Know About Health Claims on Food Labels and Dietary ... In general, health claims are statements made on food product labels or dietary supplements that boast some type of health benefit. This may seem simple, but the FDA doesn't treat every claim the same way. Label claims come in multiple forms: Health claims (which comprise of authorized health claims and qualified health claims) Introduction to Food Product Claims — FDA Reader A Qualified Health Claim is a statement approved by the FDA for use on food labels that has strict wording requirements. When there is emerging evidence between a food and the reduced risk of a disease or health condition, but not enough for the FDA to issue an Authorized Health Claim, the FDA may approve a "Qualified Health Claim". 5 Understanding Food Labels and Health Claims - Maricopa Health Claims & Foods To keep companies from making false claims, the FDA provides food manufacturers' regulations in putting labels on packages that promote health. There are three levels of health claims: A health claim is supported by scientific evidence. An example is "reduces heart disease." Drugs vs. foods - Health claims on food labels - Food labels - Canadian ... See Acceptable Disease Risk Reduction Claims and Therapeutic Claims for acceptable examples. Furthermore, Section 3, FDA, prohibits the sale of a food that is labelled or advertised to the general public as a treatment, preventative or cure for any of the diseases referred to in Schedule A. Previous Table of Contents Next
Qualified Health Claims | FDA - U.S. Food and Drug Administration Food manufacturers can petition the agency to consider exercising enforcement discretion for the use of a qualified health claim. The FDA does not "approve" qualified health claim petitions.
Food Labeling & Nutrition | FDA Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Nutrition labeling for raw produce (fruits and vegetables) and...
Label Claims for Conventional Foods and Dietary Supplements there are three ways in which fda exercises its oversight in determining which health claims may be used on a label or in labeling for a conventional food or dietary supplement: 1) the 1990...
Factual Food Labels: Health Claims - University of Texas at Austin Generally, these labels are found on the front side of the food package in emphasized lettering. Health Claims. In 1990, the Nutrition Labeling and Education Act allowed claims that related a specific food component (e.g., oats) to lowered disease risk (e.g., reduced cholesterol) to be printed on the label of a food product. For example, if a ...
Nutrient Content Claims | FDA - U.S. Food and Drug Administration Nutrient Content Claims. See Claims That Can Be Made for Conventional Foods and Dietary Supplements for definitions of claims. Final Rule: Food Labeling: Nutrient Content Claims; Alpha-Linolenic ...
Legal Guide to Health Claims on Food | Law@Dayton The Nutrition Labeling and Education Act, which amended the FD&C Act in 1990, requires most foods to be labeled with serving sizes and specific nutrition information, and it sets standards for food labels that make certain health claims. The Fair Packaging and Labeling Act of 1966 spells out packaging requirements for food and other packaged ...







Post a Comment for "40 fda health claims on food labels"